Ophthalmic medications must meet particulate matter test requirements. Particulate matter may come from foreign or product-related substances. Clinical exposure to unwanted particulate matter can cause a biological response in patients including blocking capillaries and arteries, introducing microorganisms and causing an infection, or causing an allergic reaction.
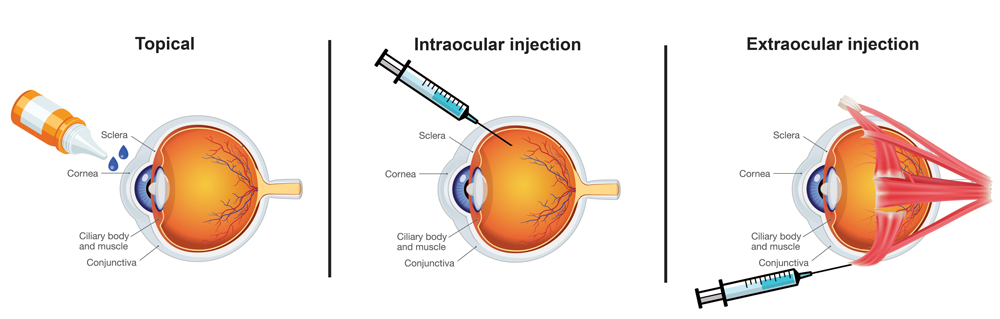
Ophthalmic products fall into three route of administration categories:
- Topical
- Intraocular Injections
- Extraocular Injections
USP <771> Ophthalmic Product Quality Tests states "all ophthalmic products should be inspected for package integrity, and to the extent possible, for the presence of observable foreign and particulate matter (visible particulates)". USP <771> also establishes subvisible particulate matter limits based on two categories for product administration to the eye:
| <789> Particulate Matter in Ophthalmic Solutions | Intraocular administration which includes all ophthalmic products that cross (penetrate) boundary tissue, such as the cornea and sclera |
| <788> Particulate Matter in Injections | Extraocular administration which includes all other ocular components and spaces (Includes Topicals and Extraocular Injections) |
Ophthalmic preparations that are suspensions, emulsions, or gels are exempt from USP <789> requirements, as are medical devices.
Once the appropriate test method has been selected, procedures for the determination of particulate matter require that an ophthalmic solution must first be tested by the light obscuration procedure (Method 1). If it fails to meet the prescribed limits, it must pass the microscopic procedure (Method 2) with its own set of test limits. Where the ophthalmic solution cannot be tested by light obscuration, microscopic testing may be used exclusively if the light obscuration procedure has been demonstrated incapable of testing the solution or it produces invalid results.
Limits for Light Obscuration and Microscopy
For intraocular use - USP <789>:
| Method | ≥10 µm | ≥25 µm | ≥50µm |
|---|---|---|---|
| 1 | NMT 50 particles/mL | NMT 5 particles/mL | NMT 2 particles/mL |
| 1 | NMT 50 particles/mL | NMT 5 particles/mL | NMT 2 particles/mL |
For extraocular use - USP <788>:
| Method | Sample Volume | ≥10 µm | ≥25 µm |
|---|---|---|---|
| 1-a | >100mL | NMT 25 particles/mL | NMT 3particles/mL |
| 1-b | ≤100mL | NMT 6000 particles/container | NMT 600 particles/container |
| 2-a | >100mL | NMT 12 particles/mL | NMT 2particles/mL |
| 1-b | ≤100mL | NMT 3000 particles/container | NMT 300 particles/container |
ARL recommends clients specify the route of administration when submitting ophthalmic injections for particulate matter testing. If the route of administration is not included on the sample submission form, ARL will test the injection by the most stringent conditions - USP <789>.
For more information on particulate matter testing, contact ARL at 800-393-1595 or info@arlok.com.
Resources:
- USP <1> Injections and Implanted Drug Products (Parenterals) - Product Quality Tests
- USP <771> Ophthalmic Products Quality Tests
- USP <788> Particulate Matter in Injections
- USP <789> Particulate Matter in Ophthalmic Solutions
- USP <1788> Methods for the Determination of Particulate Matter in Injections and Ophthalmic Solutions
